Thank you for your interest in this study.

Thank you for your interest in this study. We are looking at the amount of alcohol that people from the general population drink, as well as the practical feasibility of collecting this information. This will be achieved by the collection of blood samples from volunteers and measuring the amount of phosphotidylethanol (PEth), an established indicator of alcohol consumption, which remains in the blood for a longer period than alcohol itself.
The investigator is being paid by the sponsor (the company paying for this study) to conduct this research study.
You must be honest with the investigator about your health history or you may harm yourself by participating in this study.
Taking part in this study is entirely voluntary and you can drop out at any time you wish to with no costs or consequences (although please let us know using the email below).
Am I eligible to participate in the study?
People who are aged 21 and above, are located in the Seattle area of the United States of America, are not pregnant, or breastfeeding and weigh at least 110 pounds (50 kg) are allowed to take part. Participants must have access to the internet. Prisoners and those with diminished decision-making capacity, or a member of a vulnerable group are not able to take part in the study.
What will happen if I participate in the study?
-
-
- You will set up appointments at our clinic in Seattle for your blood samples to be collected.
- A blood sample will need to be provided on 3 separate occasions, approximately 10 days apart. This will use a device (Figure 1) that is placed on your upper arm, as illustrated below and in the video available via this link (https://www.tassoinc.com/tasso-m20-video). This process should take approximately 10 minutes on each of the 3 blood sampling occasions. The amount of blood collected on each occasion will be a few drops (approximately 100 µL).
-
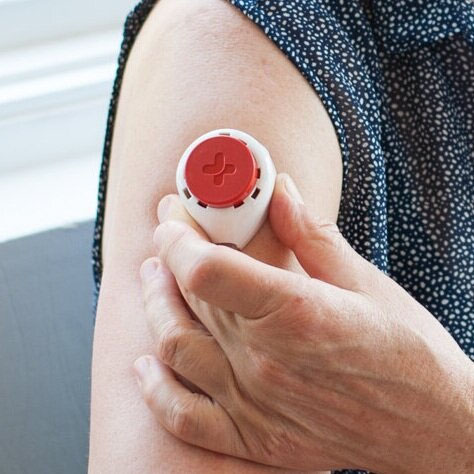
-
-
- At the end of the study, you will be e-mailed a brief questionnaire on your experiences. This questionnaire should take no more than 5 minutes to complete.
- The results of your tests will be available through either, a confidential website using a unique code, or via an e-mail. It may take up to 2 months after the last sample is collected to get the results back to you, because we are analyzing all the samples together at the end of the study. Please be assured that the results will be returned and so you will get to find out what your own alcohol consumption is and how this compares to other participants in the study.
-
What will happen to my information?
The data collected will be the information you enter into the system, as well as your test results. The people who will see this information will be restricted to yourself and the research team. If results from the study are published, this will be done on grouped data across all participants and there will be no way for someone to identify you.
Your blood samples will be destroyed after the end of the study and no genetic information will be collected from them.
Will I be compensated for taking part?
There will be three in-person visits required for this study. You will be paid $10.00 for the first in-clinic blood collection at Tasso, and a gift card will be sent to you by email within 48 hours of your Tasso visit. You will be paid $10.00 for the second in-clinic blood collection at Tasso, and a gift card will be sent to you by email within 48 hours of your Tasso visit. Finally, you will be paid $10.00 for the third in-clinic blood collection at Tasso, and a gift card will be sent to you by email within 48 hours of your Tasso visit.
The study itself should not cost you anything. However, if you need to drive anywhere because of your involvement in the study, or incur costs in any other way, these will not be reimbursed.
There is no commitment to provide any compensation for research-related injury while taking part in this study.
If you have any questions, or want extra information please just let us know: info@alcoholconsumption.study.
What are the benefits and risks of taking part?
You will get a unique and confidential insight into your own alcohol consumption that you can use to support your own healthcare.
Taking the blood sample may cause some discomfort, sensitivity and / or a small mark at the sampling site on the upper arm.
LEGAL RIGHTS
You will not lose any of your legal rights by signing this consent form.
ALTERNATIVES TO PARTICIPATING IN THE STUDY
Since this study is for research only, the only other choice would be not to be in the study.
WHOM TO CONTACT ABOUT THIS STUDY
During the study, if you experience any medical problems, suffer a research-related injury, or have questions, concerns or complaints about the study such as:
• Whom to contact in the case of a research-related injury or illness;
• Payment or compensation for being in the study, if any;
• Your responsibilities as a research participant;
• Eligibility to participate in the study;
• The investigator’s or study site’s decision to withdraw you from participation;
• Results of tests and/or procedures;
Please contact the investigator at the telephone number listed below.
| Sponsor / Study Title: | GSA Research / “A Study to Understand the Influence of Blood Sampling Approaches on Clinical Trial Recruitment and Retention Based on Sample Collection in a Clinical Setting, or in the Home” |
| Protocol Number: | PCSIG-001 |
| Principal Investigator: | George Atiee, MD |
| Telephone: | 210-419-4226 (24-Hour) |
| Address: |
Tasso Inc. 1631 15th Ave. W, Suite 105 Seattle, WA 98119
|
If you seek emergency care, or hospitalization is required, alert the treating physician that you are participating in this research study.
An institutional review board (IRB) is an independent committee established to help protect the rights of research participants. If you have any questions about your rights as a research participant, contact:
By mail:
Study Subject Adviser
Advarra IRB
6100 Merriweather Dr., Suite 600
Columbia, MD 21044
or call toll free: 877-992-4724
or by email: adviser@advarra.com
Please reference the following number when contacting the Study Subject Adviser: Pro00052855.

