Frequently Asked Questions
What is the study about?
Thank you for your interest in this study. We are looking at the amount of alcohol that people from the general population drink, as well as the practical feasibility of collecting this information. This will be achieved by the collection of blood samples from volunteers and measuring the amount of phosphotidylethanol (PEth), an established indicator of alcohol consumption, which remains in the blood for a longer period than alcohol itself.
Taking part in this study is entirely voluntary and you can drop out at any time you wish to with no costs or consequences. Although, please let us know by emailing us at info@alcoholconsumption.study.
Am I eligible to participate in the study?
People who are aged 21 and above, are located in the Seattle area of the United States of America, are not pregnant, or breastfeeding and weigh at least 110 pounds (50 kg) are allowed to take part. Participants must have access to the internet. Prisoners and those with diminished decision-making capacity, or a member of a vulnerable group are not able to take part in the study.
What will happen if I participate in the study?
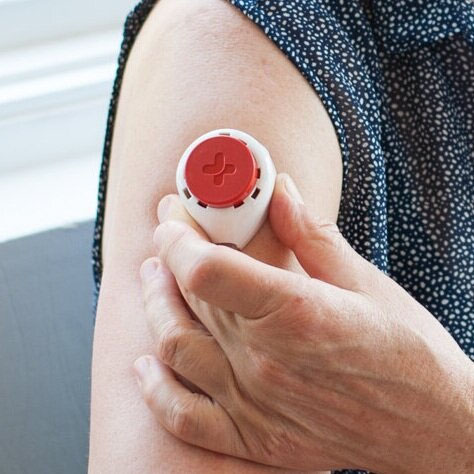
- A blood sample will need to be provided on 3 separate occasions, approximately 10 days apart. This will use a device that is placed on your upper arm, as illustrated (Figure 1) and in the video (below). This process should take approximately 10 minutes on each of the 3 blood sampling occasions. The amount of blood collected on each occasion will be a few drops (approximately 100 μL).
- The collection of the samples will be entered by you in a secure system designed for this purpose.
- Samples will then be sent to the research team using pre-paid packaging.
- You will be asked to complete a short (up to 5 minute) questionnaire via the study app each time you give a sample.
- The results of each of your three tests will be available in the study system and a notification will be sent to tell you when they are there.. It may take up to 2 months after the last sample is collected to get the results back to you because we are analyzing all the samples together at the end of the study. Please be assured that the results will be returned and so you will get to find out what your own alcohol consumption was and how this compared to other participants in the study.
What will happen to my information?
The data collected will be the information you enter into the system, as well as your test results. The people who will see this information will be restricted to yourself and the research team. If results from the study are published, this will be done on grouped data across all participants and there will be no way for someone to identify you.
Your blood samples will be destroyed after the end of the study and no genetic information will be collected from them.
Will I be compensated for taking part?
You will not be compensated for taking part. The study itself should not cost you anything. However, if you need to drive anywhere because of your involvement in the study, or incur costs in any other way, these will not be reimbursed.
There is no commitment to provide any compensation for research-related injury while taking part in this study.
If you have any questions, or want extra information please just let us know by emailing us at info@alcoholconsumption.study.
What are the benefits and risks of taking part?
You will get a unique and confidential insight into your own alcohol consumption that you can use to support your own healthcare.
Taking the blood sample may cause some discomfort, sensitivity and / or a small mark at the sampling site on the upper arm.
Legal rights
You will not lose any of your legal rights by signing this consent form.
Alternatives to participating in the study
Since this study is for research only, the only other choice would be not to take part in the study.
Contact Information
If you have questions, concerns, or complaints about this study or to report a study related injury, contact:
George Atiee, M.D.
+1 (210) 419-4226 daytime & after hours number of the investigator
If you are unable to reach anyone at the number(s) listed above and you require immediate (life threatening) medical attention, please go to the nearest emergency room.
If you have concerns or complaints about the research, or wish to ask questions about your rights as a study subject and you do not want to talk to the investigator or study staff, you may contact Advarra IRB. Advarra IRB is a group of people that has reviewed this research study. The main goal of this review is to protect the rights and well-being of the human subjects participating in research studies. Advarra IRB’s policy indicates that all concerns/complaints are to be submitted in writing for review at a convened IRB meeting to:
Mailing address:
Study Subject Adviser
Advarra IRB
6100 Merriweather Dr., Suite 600
Columbia, MD 21044
Email address
adviser@advarra.com
If you are unable to provide your concerns/complaints in writing or if this is an emergency situation regarding subject safety, contact our office at:
877-992-4724
Please reference the following number when contacting the Study Subject Adviser: Pro00052855.
Advarra IRB has approved the information in this consent form and has given approval for the investigator to do the study. This does not mean Advarra IRB has approved your being in the study. You must consider the information in this consent form for yourself and decide if you want to be in this study.

